CAR T-cell therapy has already given years of life to cancer patients who would almost certainly have died just a few years ago – and the research is still in its infancy, said Dr. Carl June, a renowned cancer researcher from the University of Pennsylvania.
But the treatment is already creating ethical dilemmas as society determines who can benefit from the therapy and who will pay.
Time magazine in 2018 named June one of the world’s 100 most influential people. That essay was written by the now-teenage girl who had not been expected to live before he treated her with CAR T therapy.
In a session with National Press Foundation fellows, June (bio, Twitter) gave a detailed tutorial on CAR T-cell therapy, from its earliest development to the 2017 decision by the U.S. Food and Drug Administration to approve the first-ever CAR T-cell therapy. That therapy, marketed by Novartis as Kymriah, is approved to treat children and young adults with an aggressive type of leukemia. It has been given to more than 300 patients but costs more than $400,000 per year.
“Now, for the first time, there are a variety of therapies that will be curative,” said June. “You only take them once. But it costs a lot of money to make it.”
The cost of the various drugs – which ranges from more than $200,000 to as much as $1 million annually per patient – poses financial and ethical dilemmas for patients, policymakers, insurers and society at large.
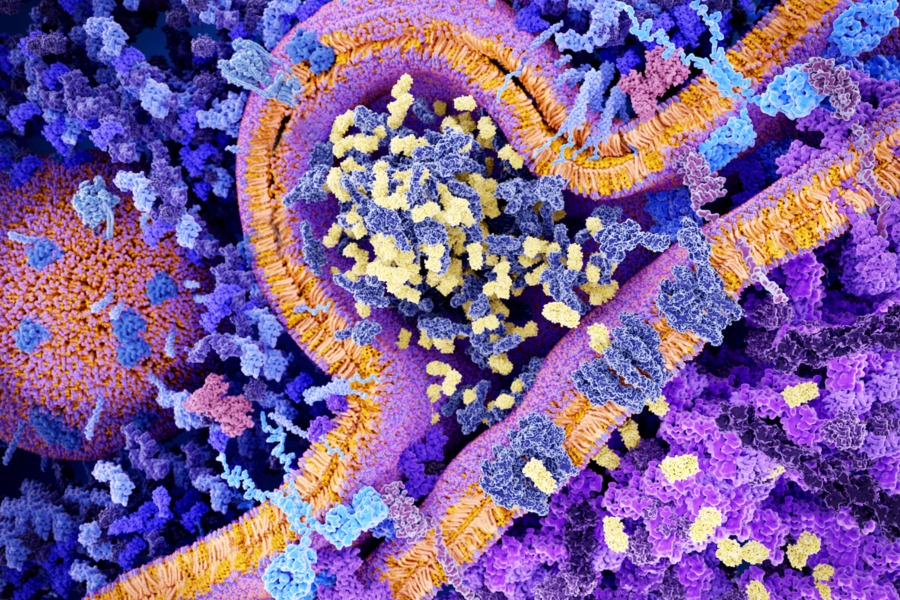
CAR T-cell therapy is a form of immunotherapy that the National Cancer Institute refers to as the “fifth pillar” of cancer treatment (after surgery, chemotherapy, radiation and targeted therapies). CAR T-cell therapy takes a patient’s T cells, modifies them in a lab to prompt them to recognize the specific cancer, and then injects them back into the patient. The acronym stands for “chimeric antigen receptor,” named for the chimera in Greek mythology, a fire-breathing hybrid creature composed of the parts of more than one animal.
The drug causes serious side effects as it attacks the cancer cells, and therefore is only approved as a last-resort therapy that hospitals and doctors must be certified to administer. And, June said, there is no financial model yet for how to pay for it.
“This will be hard to integrate into our third-party payment health system,” he said.
This program is funded by the American Association for Cancer Research. NPF is solely responsible for the content.